LIB Recycling Pre-Treatment: Sorting & Zero Discharge Approaches
Following on from our successful 'Gigafactory In-house Battery Recycling Virtual Meet' session in May '24. Today's article focusses on electric vehicle LIB battery 'Recycling Pre-Treatment: Sorting & Zero Discharge' stages as listed in the event's outputs. More information on the various available recycling technologies can be found via our website.
The key pre-treatment steps prior to recycling include:
Sorting
Zero Discharge
Dismantling
Crushing / physical separation with safety controls (recycling facility external to building)
Battery sorting and zero-discharge are the first pre-treatment stages in the recycling process of spent LIBs and enables safe handling, storage, transportation, and recycling of the spent batteries. Moreover, it helps reduce scrap volumes and allows for the separation of battery components. After pretreatment, the recovered active cathode material will be subjected to pyro-metallurgical or hydro-metallurgical processes to extract the high-value metals. (1) Thermal pre-treatment methods remain popular followed by a hybrid recycling approach which includes both pyrometallurgy and a final hydrometallurgical refining step.
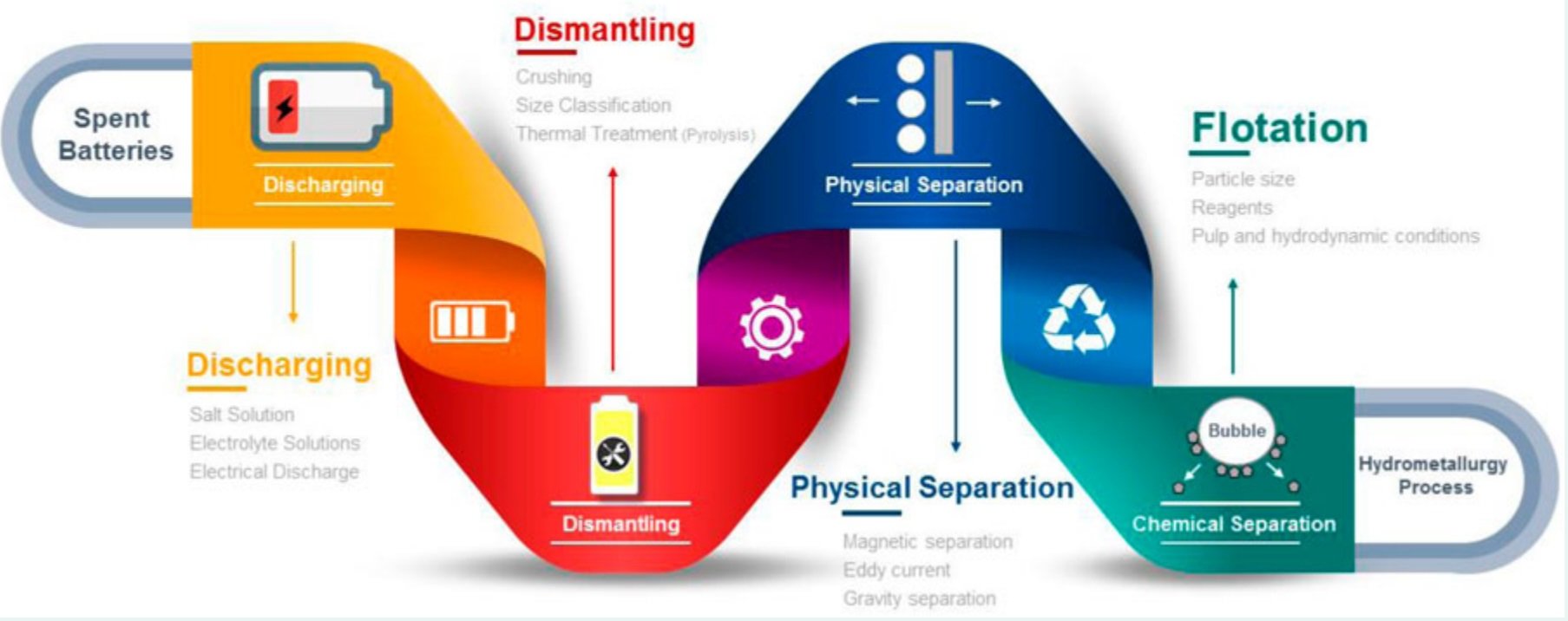
A typical pre-treatment process flow is illustrated below. (2)
Sorting
Sorting is essential not only to sort out non-battery waste but also to separate between battery chemistries, shapes and sizes according to the criteria dictated by the final recycling facility. Consideration should also be given to any size limitations of the processing equipment. Likewise, precise sorting helps to enhance the final product uniformity and quality, plus enables the targeted recycling of individual components - where valuable materials, such as steel, copper, aluminum, selected plastics, and precious metals can be recovered from the housing, cable harness, cooling system, or other electronic parts. (1)(3)
Battery sorting is also done according to the battery State of Health (SoH). Before battery deactivation, especially when using electrical discharge methods, information about the LIBs current range, remaining voltage, capacitance, state of charge must be known.
LIB sorting can be conducted manually or automatically, and multi-stage sorting can be adopted to enhance efficiency. Although several automated sorting methods such as optical sensors and X-ray sensors have been developed, the non-homogeneity of the battery structure can make a fully automated sorting process challenging to achieve, thus manual sorting is commonly employed because of its flexibility - as is often the case in third-party material recovery facilities (MRFs) (1)
Zero Discharge
To reduce thermal runaway of LIBs during disassembly, and to secure the safety of personnel, it is necessary to discharge LIBs prior to dismantling. In the event sparks are produced, they might cause the ignition of volatile organic compounds during the downstream mechanical pre-treatment process. Also, the state of charge (SoC) should ideally be 0% (corresponding to a voltage of approx. 2V). (4)
Discharging can be performed by different methods. Several companies use thermal pre-treatment (e.g., Accurec or REDUX in Germany), salt-water based baths (NaCl or Na2SO4), or controlled discharging via external circuits. In some cases, batteries are discharged via cryogenic methods using liquid nitrogen or in vacuum atmospheres. Such methods are OPEX / CAPEX intensive. (5)
Some schools of thought deem the discharging step unnecessary for industrial-scale operations when shredding LIBs. However, if they are shredded without discharging, it is important to perform the process under a well-ventilated system to collect the gases released. (2) Also, the complete discharge of cells to 0V will cause the Cu foil to oxidize on the anode and dissolve into the electrolyte, then further deposit on the cathode, contaminating the cathode unnecessarily. (1)
Discharging via Load Banks
Electrical discharge is a proven method that has been industrialised, and is mainly used to test batteries and data banks rather than for discharge purposes.
Typical parameters required for the design & selection of 'Battery Discharge Load Banks' include the 'rated voltage and continuous power' in order to calculate the 'resistance value of the load'. The resistance will need to reduce in proportion with the voltage in order to continue to draw current and discharge the battery. To achieve the final, deep discharge - a very low resistance load to draw the final current from the battery is needed and to sustain this for sufficient time to ensure the battery voltage does not increase after the load is disconnected. Thus, an element of power monitoring and control logic is required too. Cressall Resistors Ltd, (based in the UK), have the capabilities to develop customised solutions for clients once the above parameters are defined.
Battery discharging can be carried out electrically using dynamic or static resistance. An electronic load is typically applied to offer the necessary resistance, with the energy dissipated as heat. Modern load banks can be “regenerative” in nature and thus integrate with an AC/DC inverter - enabling recovery of the energy as alternating current (AC). Although 'regenerative’ load banks are more expensive than conventional load banks, they offer energy conservation potential up to 80–94.5% of the battery energy. Companies including Duesenfeld and TES-AMM are said to have trialled the coupling of regenerative load banks to commercial energy storage systems. (6)
The efficiency of regenerative banks is highest when the energy content of the spent LIB is high - conversely, little to no energy recovery is possible for over-discharged batteries (0–2.5 V). Due to variations in battery design, automation can be challenging, coupled with the safety risks associated with manually connecting between the given battery and the load.
As discussed in the 'sorting' section - information about the current range, allowed voltage, capacitance, state of health, and charge must be acquired prior to discharge to ensure a safe discharge process - which all equates to longer processing times. (1) Likewise, the time constraints may make this method impractical in an industrial setting. Key variables that govern this activity include the battery’s capacity (Ah), the discharge rate (C-rate), and the load applied. Finally, the current in the electric circuit must be closely monitored to mitigate the fire risk. (4)
Other safety considerations include dealing with batteries with disconnected electrical circuits, either due to the operation of safety devices such as current interrupt devices or deposited Li, which is often electrically disconnected from the electrode. If such LIBs are sent for mechanical treatment without being sufficiently deactivated, hazards may arise due to their residual voltage. Therefore, such LIBs can only be rendered safe through roasting (see 'Pyrolysis for deactivation section'), resulting in increased CO2 emissions and reduced recycling efficiency. (7)
Below is a selection of suppliers who provide off-the-shelf and tailored EV battery discharge equipment.
Cressall Resistors Ltd, as mentioned above.
Elektro-Automatik, (acquired by Tektronix based in the UK), offers a Battery Simulator Series EA-BT 20000 Series | Tektronix that is able to recover energy from batteries at their end of life before being sent to recycling. An integrated 'SOH' function followed by an 'auto ranging' capability allows the maximum possible discharge current down to 2 V while the regenerative capability feeds recovered energy back into the grid with 96% efficiency. More information is available via their website: EV Battery Testing, Charging & Simulation | Elektro-Automatik (elektroautomatik.com).
Chromaeu, a Dutch based company, also offer various options, both via battery system solutions and electronic loads, regenerative or non-regenerative. They are able to conduct measurements and capacity calculations during discharge. The battery test solutions include the 17020 and 17040 series. The E-load solutions include the 63700-series (regenerative loads) or 63200-series (non-regenerative). By connecting them in parallel, the 63700 series can reach a maximum power of 180kW, making it suitable for power requirements ranging from 5kW to 180kW.
Likewise, the 63700 features an energy recovery function and has external protection mechanisms. When the 63700 detects AC input over voltage (OV) or under voltage (UV), abnormal frequency (Freq. Error), three-phase imbalance (Unbalance), or over current (OC), it will shut off the module power to ensure safe grid integration.
WinAck, a Chinese based manufacturer, also offer two types of battery discharge equipment. One is the traditional resistive discharge type, which simply converts electrical energy into heat energy and then dissipates it. The other is the regenerative type, which can regenerate battery discharge energy to the AC power grid for use by other devices. The resistive offering is suitable for batteries with a maximum voltage of ≤800V and can discharge the battery to a minimum of 10V. Voltage range, current range and discharge power can also be customised according to requirements.
Recent research suggests the optimal temperature to facilitate discharge to be at 35 ◦C. At this temperature, the amount of remaining energy in a fully discharged LIB is significantly reduced, which reduces the risk of thermal runaway and fire or explosion during the recycling process. Conversely, if the battery pack is discharged at a lower temperature, the internal resistance of the battery increases, causing the battery voltage to drop at a slower rate. (4)
Discharging via Salt-Water Baths
Salt-water based discharge is a flexible and comparably safe way to stabilise different types of high-energy cells. The rate of discharge varies depending on several factors, but predominantly on the solution resistance—itself depending on the conductivity of the solution and the distance between the electrodes.
NaCl is typically reported as having the best discharge profile and capability to fully discharge batteries. (5) The main problem faced during battery discharge is the deposition of sediments and electrode corrosion. Temperature variations typically do not yield significant differences. However, with the use of ultrasonication, the discharge time can be reduced to less than 2 hours. The ultrasonic effect also overcomes the sedimentation issue described above. Other side-effects to be aware of include the potential generation of hydrogen & chlorine gas during the process, plus the leaching of Li or Cu components into the brine water due to corrosion of the battery case. Also, a variety of reagents have been explored to help speed up discharge times, each with their own disadvantages that hamper industrial scale use. Alternative configurations may also reduce discharge times further (i.e. vertical immersion), also reducing sedimentation and corrosion issues. Finally, consideration should be given that post-treatment of the brine water is required once spent. (1)(6)(7)(8)
Discharging via Cryogenic Processes
Recent advancements have seen cryogenic freezing being employed to deactivate the spent LIBs, and it has been adopted by numerous industrial recycling companies such as Umicore and Retriev (formerly Toxco). The spent LIBs are subjected to cryogenic temperatures (− 175 ℃ to − 200 ℃), usually in liquid nitrogen / N2 & O2 mixtures to freeze and crystalize the electrolyte. The LIB becomes non-conductive, thereby attaining the temporary inactivation of the spent LIBs, which has the same effect as discharging them. The low temperature used in cryogenic cooling makes lithium inert, eliminating the fire risk caused by elemental lithium oxidation. This method is suitable for processing high-capacity batteries and can process large quantities of spent LIBs simultaneously. However, it has high capital and equipment requirements thus hampering industrial scale feasibilities. A typical follow-on treatment process includes cryogenic grinding (known as freezer milling). (1)(2)(5)
Pyrolysis for Deactivation
Thermal deactivation has been adopted by some industrial recycling facilities (I.e. Accurec). This method offers complete deactivation of the LIB cells to prevent sudden thermal events by removing the high energy and organic content of the spent LIBs in a controlled manner. The detailed procedure entails manually sorting the Li-consumer type batteries into subtypes and subjecting the batteries to vacuum thermal recycling (VTR) (temperature ≤ 250 ℃). The organic components (electrolyte, plastics, binders) are removed entirely by pyrolysis during VTR, and the state of the metal contents remains unchanged. The LIBs are discharged during this VTR process as the electrolyte evaporates. The electrolyte is condensed in a condenser system, and the condensate composition is ~10% ethylene carbonate and ~71% ethyl methyl carbonate, but it is nearly impractical to reuse it as it contains decomposition products. The condensate recovery can be made in a separate process with up to 80% recovery rate. Approximately 12% of lithium is lost by evaporation to the off-gas, and fractional graphite is also lost during the VTR process. Thermal deactivation offers the advantage of controlled deactivation and the safe destruction of combustible organic material, eliminating the risk of fluorine compounds being released into the atmosphere or electrolyte reacting with the atmosphere during mechanical pretreatment. The process incorporates a high-end particle filtering system (HEPA), and thus there is no emission of toxic gases or metals. (1)
This method has traditionally been considered for recycling LIBs with high Ni and Co concentrations since it is easy to operate. Alternative heating regimes include heating the spent LIBs to 100–150 °C for 1 h [28–31] or 400 °C for 17–20 min to discharge them before any pretreatment steps. Thermal discharging is irreversible and can damage the electrolyte and release hazardous gas. (2)
Depending on the active material, it is advisable to discharge the cells first. The active material based on LFP-type cathode materials shows a low reaction and does not need to be discharged in advance while those based on LMO- or NMC type cathodes are much more reactive and should be discharged before undergoing the pyrolysis process.
After pyrolysis, the batteries can be stored temporarily and, as a consequence, mechanical treatment can be carried out with low risks of fire or thermal runaways.
With regard to the furnaces themselves, they are placed under vacuum and filled with high purity nitrogen. Academic experiments of pyrolysis at 600 ◦C under atmosphere did not achieve satisfactory results, as only parts of the cathode material could be detached, while the electrode was deformed, fragile and oxidised slightly at these high temperatures. This shows that pyrolysis under vacuum is necessary if the aluminium foil should be recovered in its metallic form. (3)
Material Separation driven by Reaction Passivation
This method works on the basis of reactions between the cathode and anode, or the anode and a tailored chemical environment - facilitating rupture of the cell by way of a protective atmosphere such as N2, CO2, or CO2 and Ar. Both Akkuse and Duesenfeld have reportedly filed patents using this method.
In both patents, the anode electrode is exposed to the inert gases described thus creating a passivation layer containing a certain amount of lithium carbonate. Other patents (Retriev) include alternative passivation methods utilising water directly as a combined passivation agent, an oxygen barrier and a heat dissipator. This process allows both crushing and disassembly to be operated in water - preventing both detonation and uncontrolled energy release. Other studies include the disassembly of charged spent LIBs directly in pure water. The active lithium in the anode material reacts directly with water to passivate into lithium hydroxide, whilst the water separates the air and absorbs any heat generated to prevent thermal runaway.
Whilst this approach offers promise on a large scale, the disadvantage is it is difficult to control the desired passivation effect in high volume, rapid processes. Also, the gas or water used for passivation will inevitably contain organic matter and require post-treatment. (6)
Post-Discharge
Depending on the deactivation process used, consideration should be given to the possibility that the discharged battery voltage can gradually rise again, which induces certain risks in subsequent processing stages. (1)
Further energy recovery
It is envisioned that considerable economies of scale can be achieved through the recovery of electrical energy in decommissioned LIBs. Where resistive load bank discharging is used, the converted heat energy has potential for recovery. Likewise, saltwater discharging methods can be designed to maximise gas production - promoting recovery of i.e. oxygen and chlorine - similar to chlorine gas production via electrolysis in the water industry. Conversely, when considering controlled passivation - due to the energy release via direct reactions, energy recovery becomes difficult.
More promising approaches trialled by Duesenfeld and TES-AMM include the use of DC/DC modules or switching DC power supplies to directly transfer the energy to the local busbar or an external energy storage system - known as the 'direct energy recovery discharge mode'. (6)
We hope you found this article useful!
If you are looking for professional process engineering, feasibility study or environmental facility engineering support relating to your next industrial mega-facility design & build project...please reach out to us!
Contact — Biyat Energy & Environment Ltd (biyatenergyenvironment.com)
This article was written by Luay Zayed, Founder' of Biyat Energy & Environment Ltd. A global energy and environmental consultancy specializing in turnkey engineering solutions that protect the environment and improve energy efficiency in the manufacturing & industrial sectors.
References
(2) - Recycling spent lithium batteries – an overview of pretreatment flowsheet development based on metal (tandfonline.com) (2023)
(4) - Batteries | Free Full-Text | Pretreatment of Lithium Ion Batteries for Safe Recycling with High-Temperature Discharging Approach (mdpi.com) (2024)
(5) - Recycling of Lithium‐Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling (wiley.com) (2022)
(7) - Battery deactivation with redox shuttles for safe and efficient recycling | Scientific Reports (nature.com) (2024)
(8) - Discharge of lithium-ion batteries in salt solutions for safer storage, transport, and resource recovery (sagepub.com) (2022)
Further reading
Treatment and recycling of spent lithium-based batteries: a review | Journal of Material Cycles and Waste Management (springer.com) (2023)
(PDF) RECYCLING OF LITHIUM- ION BATTERIES (researchgate.net) (2021)
Technologies of lithium recycling from waste lithium ion batteries: a review (rsc.org) (2021)